Separation of Mixtures
Separation of Mixtures: Overview
This Topic covers sub-topics such as Chromatography, Centrifugation, Sublimation, Paper Chromatography, Fractional Distillation, Principle of Chromatography, Principle of Centrifugation, Separation of Cream from Milk and, Application of Distillation
Important Questions on Separation of Mixtures
Particles are not separated from a solution according to their shape.
What is use of density gradient centrifugation?
______ is a process used quite often in the dairy industry.
A chalk stick is dipped in ink and after breaking it, it is found to be white from inside.
Chalk _____ separates pigments in dyes based on their movement through porous chalk.
When a chalk stick is dipped in ink solvent of the ink goes deeper into the stick due to adsorption.
Describe the chromatography process using chalk.
The process in which a solid is converted to gaseous state is called _____.
What is the principle used to separate two immiscible liquids?
Two immiscible liquids are separated by distillation.
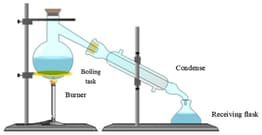
Observe the diagram carefully. Point out the place where water vapour condenses into water.
A liquid with boiling point189$ ℃$ is soluble in water.

Which of the following processes can be used to separate a colourless solution of the liquid from water?
State true or false.
The picture shows the formation of water vapour inside the condenser during the distillation of salt solution. These water vapours are free from any dissolved solid impurities.
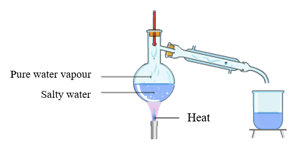
In a severe water contamination in home water supply which of the following is the recommended method for removal of soluble impurities from water?
Ink is soluble in water. How can you get pure water from a mixture of ink and water?
A mixture of two or more miscible liquids, for which the difference in the boiling points is less than 25K can be separated by the process called
Which of the following materials could be a pure substance?
Smoke coming out of the chimneys in factories is seen near the mouth. After rising high, it vanishes after mixing in the atmosphere. This happens due to the process of